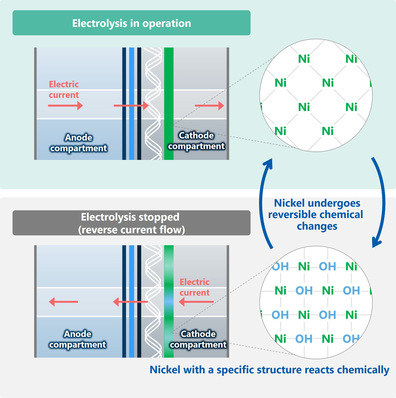
Shizhang Qiao, chemical engineer at the University of Adelaide, has explained: “We have split natural seawater into oxygen and hydrogen with nearly 100 percent efficiency, to produce green hydrogen by electrolysis, using a non-precious and cheap catalyst in a commercial electrolyzer.” If, in contrast, freshwater is used in conventional electrolyzers with their far more expensive catalysts made of platinum and iridium, hardly any less hydrogen is produced, expressed Yao Zheng, an assistant professor in materials science.
Researchers have succeeded in producing hydrogen directly from seawater
H2 from seawater

No time? No problem with the H2 International newsletter
With our newsletter, you will regularly receive selected information and news from us, bundled and free of charge directly to your mailbox.
With the subscription to this newsletter, I agree to be informed about interesting publishing and online offers of Alfons W. Gentner Verlag GmbH & Co. KG. I can revoke this agreement and unsubscribe at any time. Further information on the handling of data can also be found in our privacy policy.