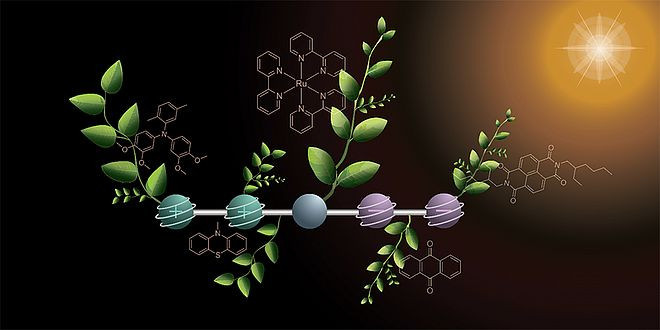
The team led by chemist Oliver Wenger from the University of Basel has developed a molecule that can simultaneously store two positive and two negative charges when exposed to light. The results were published in the journal “Nature Chemistry.” Charge storage is a key step in artificial photosynthesis, in which sunlight is converted into chemical energy – for example, to produce hydrogen.
The molecule consists of five functional units arranged in a chain. Two of them donate electrons and become positively charged, while two others accept electrons and become negatively charged. In the center is a light-active component that triggers the reaction. The researchers used two successive light pulses to generate the four charges. In the process, one positive and one negative charge are initially created and stabilized at the ends of the molecule. The second light pulse repeats this process.
Use of sunlight possible
According to Mathis Brändlin, a doctoral student on the team, the molecule can already be excited using relatively weak light. “This stepwise excitation allows us to use significantly weaker light. We are already approaching the intensity of sunlight,” says Brändlin. In addition, the generated charges remain stable long enough to be used in subsequent chemical reactions – such as splitting water into hydrogen and oxygen.
This is not yet a complete system for artificial photosynthesis. However, Wenger emphasizes the significance of the progress: “We have identified and realized an important piece of the puzzle.” The findings are intended to help improve understanding of electron transfer mechanisms and, in the long term, contribute to the development of CO2-neutral fuels.