Catalysts for AEM electrolysers (anion exchange membrane electrolysers) do not require expensive precious metals. Yet there is still scope to reduce costs further. Work is ongoing to improve the performance and durability of catalyst-coated electrodes. In addition to material suitability, researchers are conducting extensive investigations into alternative catalyst compositions and manufacturing processes.
Researchers from ZBT – Zentrum für Brennstoffzellentechnik in Duisburg, fem Forschungsinstitut in Schwäbisch Gmünd and Ruhr-Universität Bochum have made significant progress in developing cost-effective and durable anodes for hydrogen production as part of a joint project funded by the IGF (Industrielle Gemeinschaftsforschung), a German programme for pre-competitive industrial collective research. To enhance the performance and corrosion resistance of the nickel-based catalyst layer, the team incorporated non-metallic elements such as sulphur and phosphorus into the catalyst layer and formed nanocone structures in the anode layer.
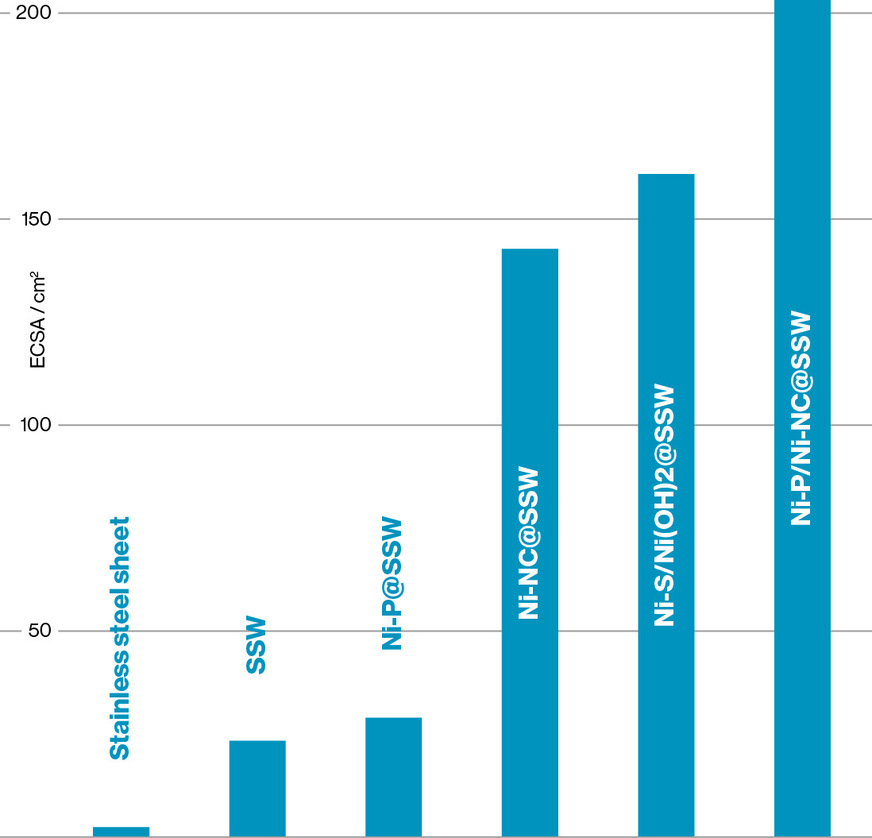
The anodes developed in the project, produced by electrodeposition onto porous stainless steel non-woven material, achieve approximately 20 percent higher current density at the same cell voltage compared to untreated stainless steel non-woven. Stainless steel already demonstrates good catalytic activity in AEM electrolysis and therefore serves as the reference.
© ZBT/NEONBOLD
Optimised surface and durability
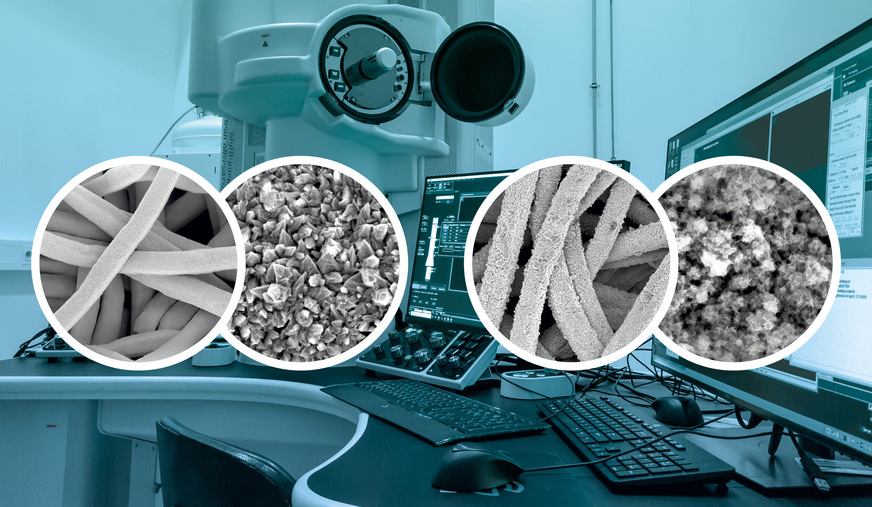
The modified anodes optimise the active surface area, thereby improving the kinetics of the oxygen evolution reaction (OER). Using scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDX), the researchers confirmed the desired nanostructured morphology and homogeneous element distribution.
In single-cell tests, they also observed superior activity of Ni-S electrodes compared to bare stainless steel. During constant current measurement at 2.5 A over ten hours, the electrolysis cell with the optimised anode started at 1.69 V, while the stainless steel non-woven required 1.92 V. This corresponds to a reduction in cell voltage of approximately 12 percent. Furthermore, pronounced corrosion was observed on the bare stainless steel non-woven after the constant current measurement, whereas the Ni-S layer remained structurally intact.
The targeted formation of lamellar hydroxide structures on the electrode surface, which are retained after electrolysis, stabilises and further enhances catalytic activity in the alkaline environment. The nanocone structures increase the effective surface area of the electrode and can serve as robust supports for active catalyst coatings, as they remain stable even after electrolysis under alkaline conditions.
Through electrochemical measurements as well as structural and chemical analyses, the researchers demonstrated that the combination of nickel and sulphur forms a particularly active and stable surface for oxygen evolution:
• Nickel hydroxide and nickel oxyhydroxide phases are known to actively participate in the OER in alkaline media – including in AEM electrolysis.
• The incorporation of sulphur modifies the electronic structure of nickel – the electron properties that influence its conductivity, binding strengths to reactants and thus its catalytic activity.
• This optimises the adsorption strength of oxygen intermediates on the catalyst surface. The binding energy is shifted towards an optimal value (neither too strong nor too weak).
• The result: lower anodic overpotential at a given current density, which translates into lower cell voltages.
© ZBT / Nikky – stock.adobe.com
Scalable manufacturing process
The key innovation for future application lies in electrochemical deposition (electrodeposition). This process allows precise control over the composition and structure of the catalyst layer. The method is also scalable and therefore industrially viable – a decisive advantage over more complex manufacturing processes.
Miriam Hesse, a doctoral researcher at ZBT and co-author of the publication, focuses her research on the production, characterisation and testing of precious-metal-free electrodes in real AEM electrolysis systems. She investigates how the composition, structure and surface morphology of catalysts influence their activity and stability. Her work includes electrochemical measurements and analyses using X-ray fluorescence (XRF), X-ray diffractometry (XRD) and scanning electron microscopy (SEM), which she performs before and after electrolysis operation to assess changes in the composition and microstructure of the materials.
The project not only demonstrates that numerous promising materials have been developed in laboratory research in recent years, but also addresses precisely this point by advancing their transfer into practical electrolysis applications. Hesse’s work thus bridges the gap between fundamental materials science research and the application-oriented evaluation of electrodes for future electrolysis systems.
The recently published study highlights the potential of electrodeposited nickel-based materials for the scalable production of efficient anodes for AEM electrolysis. It provides an important impetus for the development of cost-effective hydrogen production technologies.
About ZBT
ZBT – Zentrum für BrennstoffzellenTechnik was founded in Duisburg, Germany, in 2001 and is one of Europe's leading application-oriented research institutions for fuel cells, hydrogen technologies and electrolysis processes. ZBT is a sought-after research and development partner in European and national cutting-edge research as well as in industry projects.
From materials to components and complete systems, from green hydrogen production through refuelling to reconversion to electricity, from prototypes through small-series to mass production – ZBT bridges the gap between university-based fundamental research and industrial development departments.