Hydrogen has the potential to take a particularly important role within the technological shift away from fossil fuels towards renewable and alternative sources of energy. Plans for using renewably sourced hydrogen as an energy carrier (e.g., power-to-gas) in order to deal with periods of power oversupply have posed special challenges for the energy industry. In addition to environmental and economic considerations, the increase in the amount of hydrogen creates challenges for material use, since hydrogen may cause spontaneous failures of metallic components, a process that is known as “hydrogen embrittlement.”
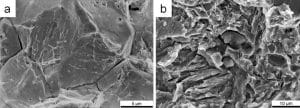
Through humid furnace lining, additives and alloying elements, hydrogen may diffuse into components or semi-finished products during metallurgical production to cause damage such as flakes or fish-eyes. There is a similar risk during welding. Most hydrogen-induced damage, however, occurs when the component has been put into operation.
If the hydrogen causing the damage enters the component during manufacturing or processing steps, e.g. pickling or galvanizing, and is then “activated” due to applied operational stresses, the mechanism is called “delayed fracture.” If the hydrogen is only introduced during use of the component, as in the case of cathodic partial reactions of corrosion processes, it is called “cathodic stress corrosion cracking.” In both cases, most of the components fail spontaneously without any prior warning such as deformation.
Atomic, trapped or molecular
Most hydrogen-induced damage is triggered by atomic (diffusible) hydrogen (H+). Because of the small size of the atoms, diffusion in steel occurs at a very high rate even at room temperature, similar to carbon at 800 °C. Externally applied stresses, which may overlap with residual stresses, will strain the metal lattice elastically, establishing the driving force for atomic hydrogen to diffuse into those areas and accumulate. This process, called Gorsky effect, is intensified by notches and cracks causing a localized stress concentration.
Conversely, so-called trapped hydrogen (H+T) accumulates at energetically favorable lattice sites, e.g. dislocations, inclusions, grain boundaries, etc. Trapped hydrogen is no longer diffusible at room temperature and usually does not pose a risk. Only by applying activation energy in the form of rising temperatures, which is characteristic for each type of trap, hydrogen can be released from the traps and diffuse into the metal lattice
…





















0 Comments